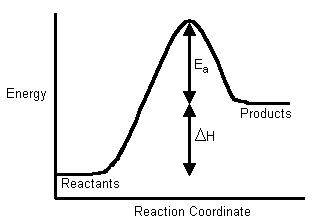
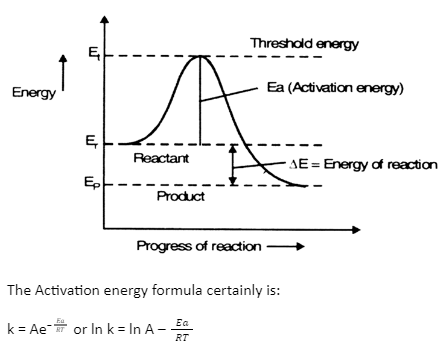
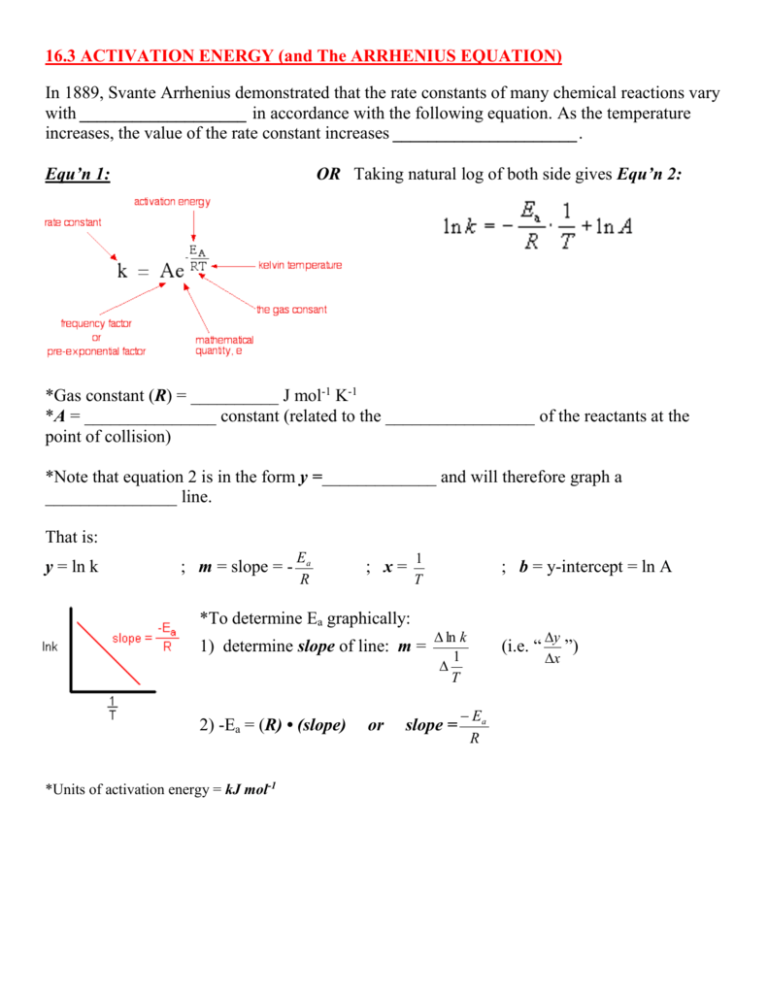
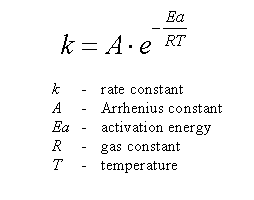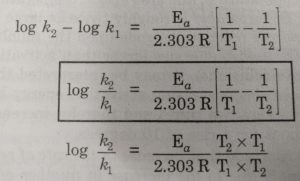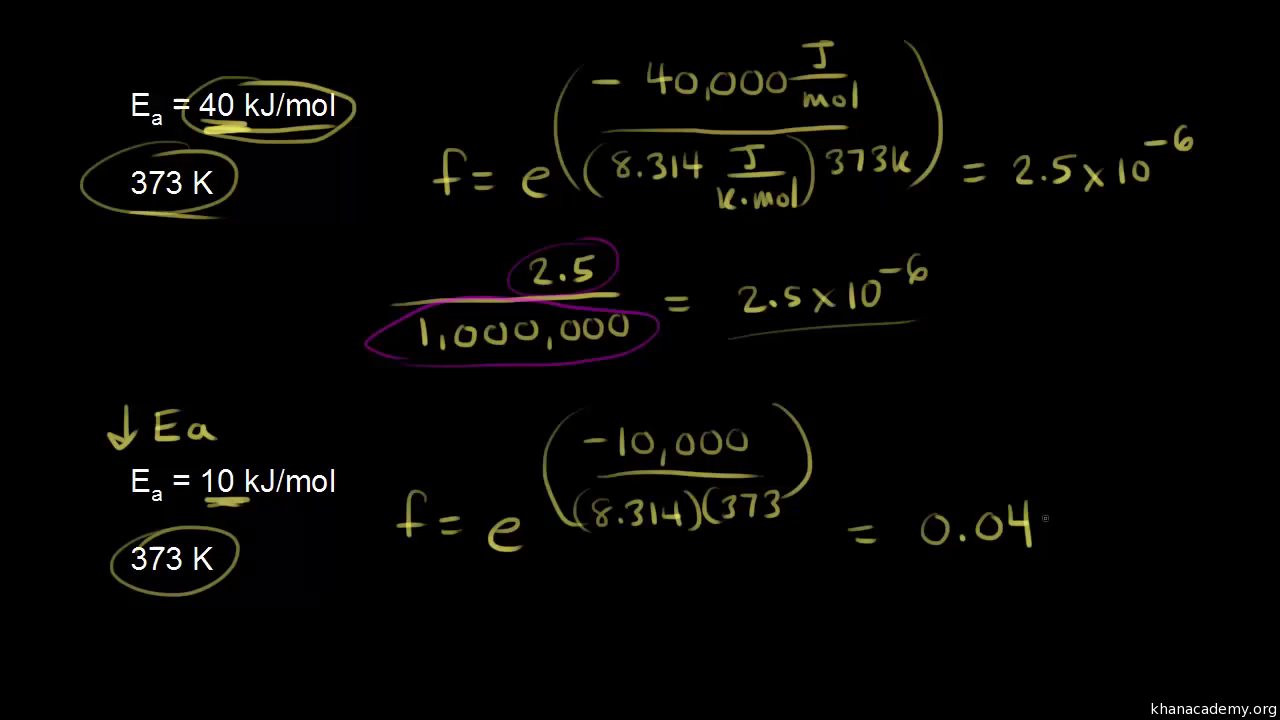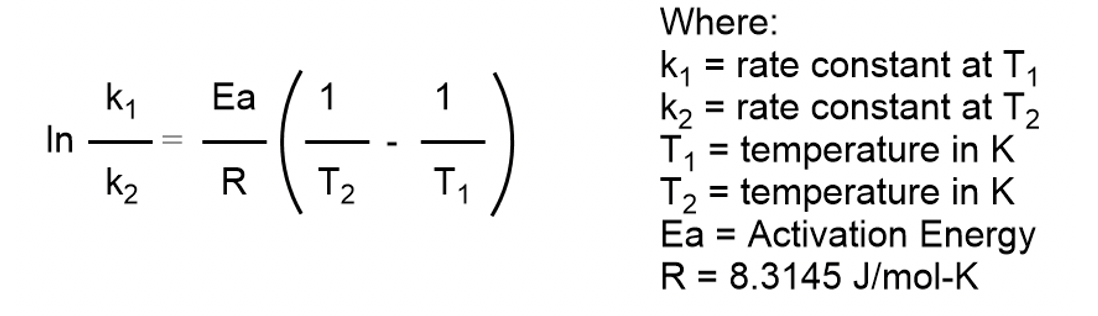SOLVED: The activation energy Ea can be defined from the Arrhenius expression as: Ea = RT^2 / (2.303 * T^2 * bmax * Vrex) To show Ea = (1/2) kT + e*,

Numerical on Arrhenius equation > The rate constant a first order reaction becomes six times when the temperature is raised from 350 to 4ook. Calculate Ea = ? Ang kesh R= 3:314J

Calculation of activation energy (Ea) for the dose function. RH = 85%. | Download Scientific Diagram
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10^ 3sec^ 1 at 40℃.calculate the energy of activation of the reaction
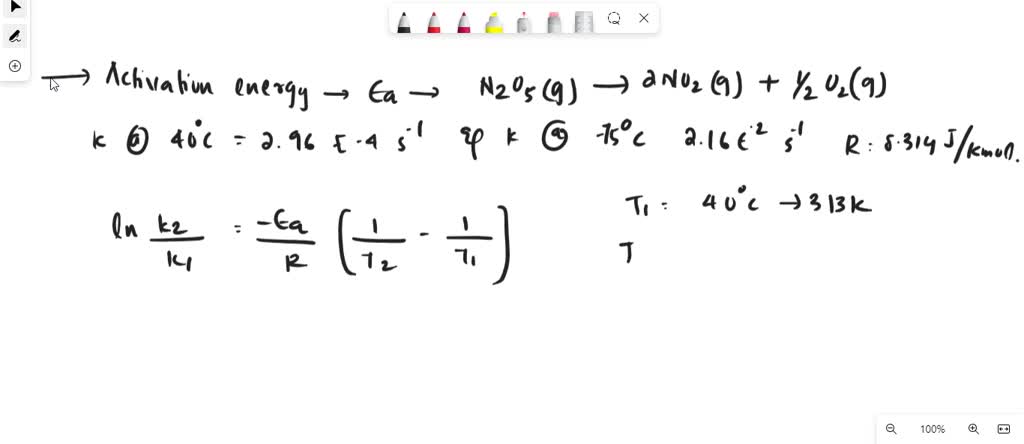
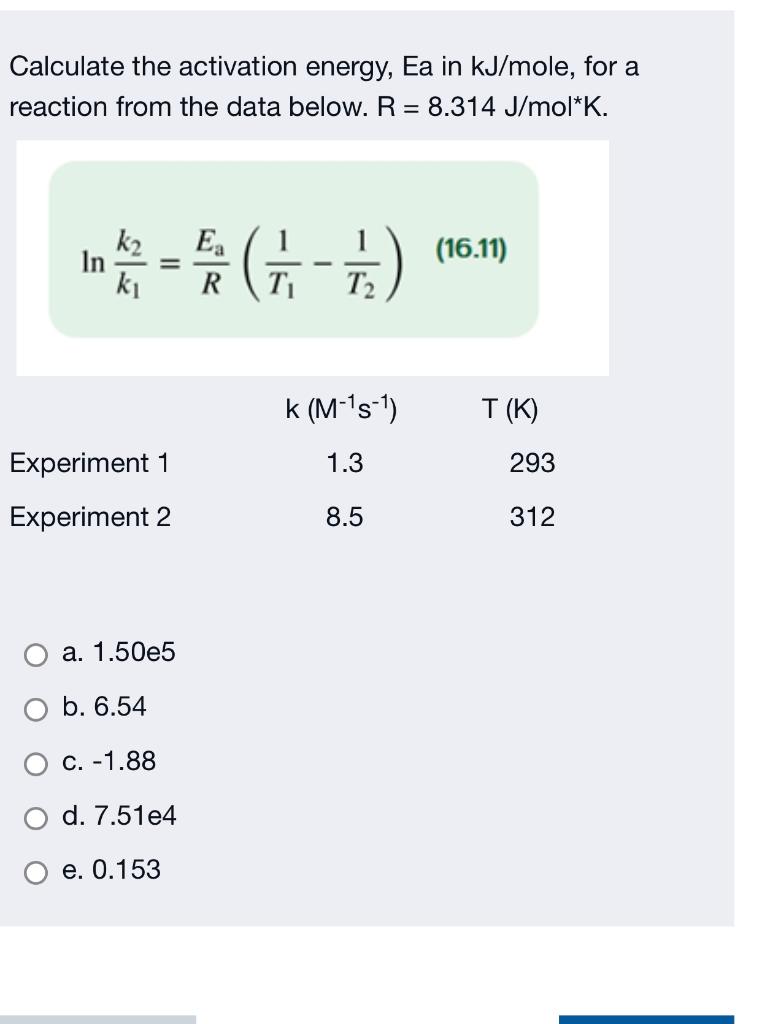
SOLVED: Calculate the activation energy, Ea, for N2O5(g) â†' 2 NO2(g) + 1/2 O2(g) given k (at 45.0 °C) = 5.79 × 10^-4 s^-1 and k (at 60.0 °C) = 3.83 ×
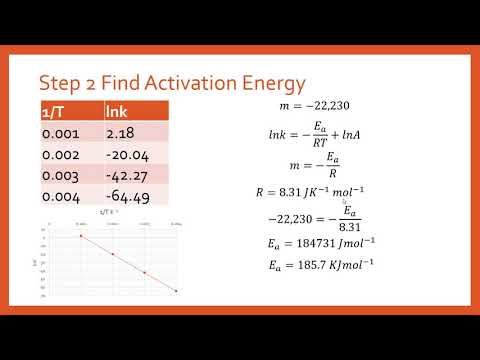
What is the activation energy for a given reaction if a plot of ln(k) versus (1/T) gives a slope of -307.4 K–1? - Quora