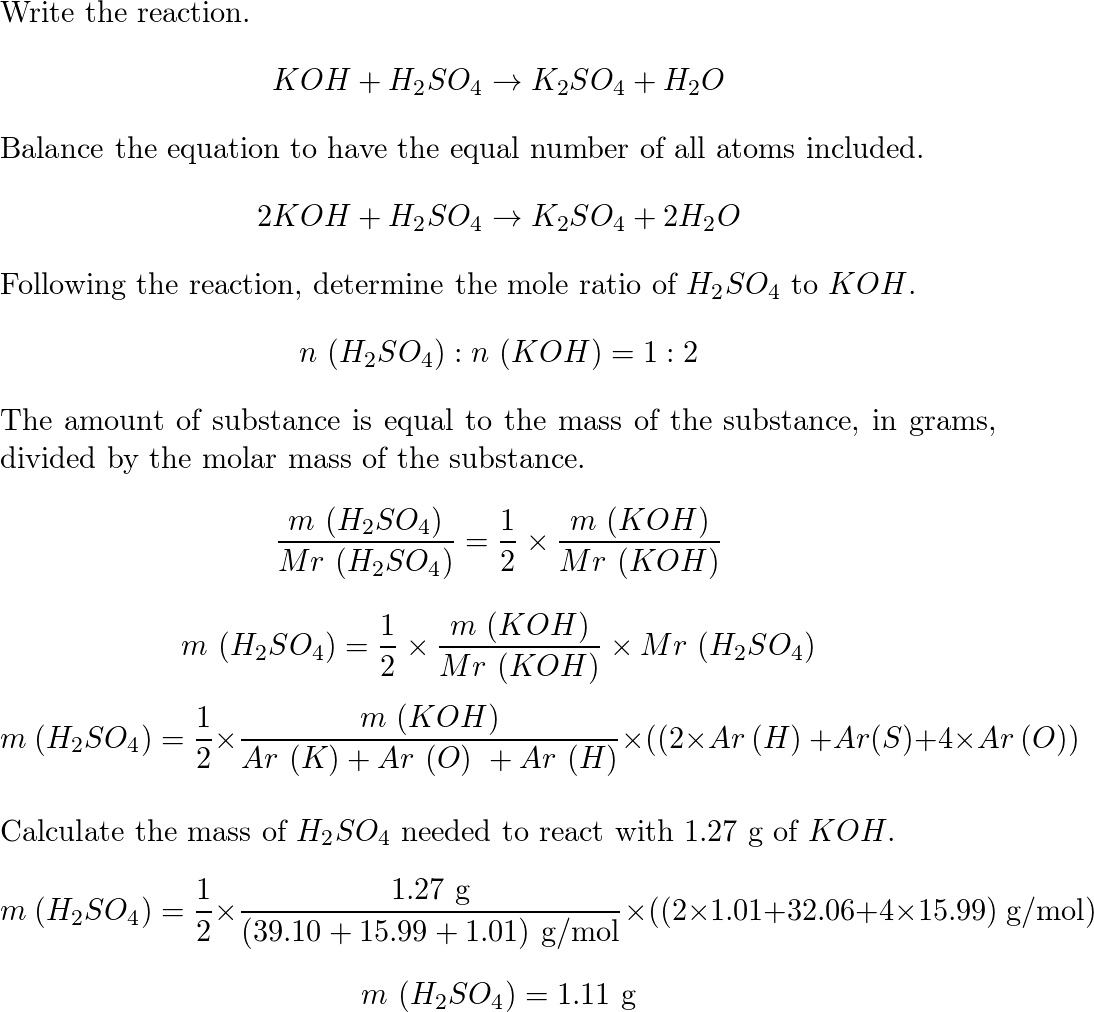Balance the following equation by oxidation number method : (i) K2Cr2O7 + KCl + H2SO4 → KHSO4 + CrO2Cl2 + H2O - Sarthaks eConnect | Largest Online Education Community
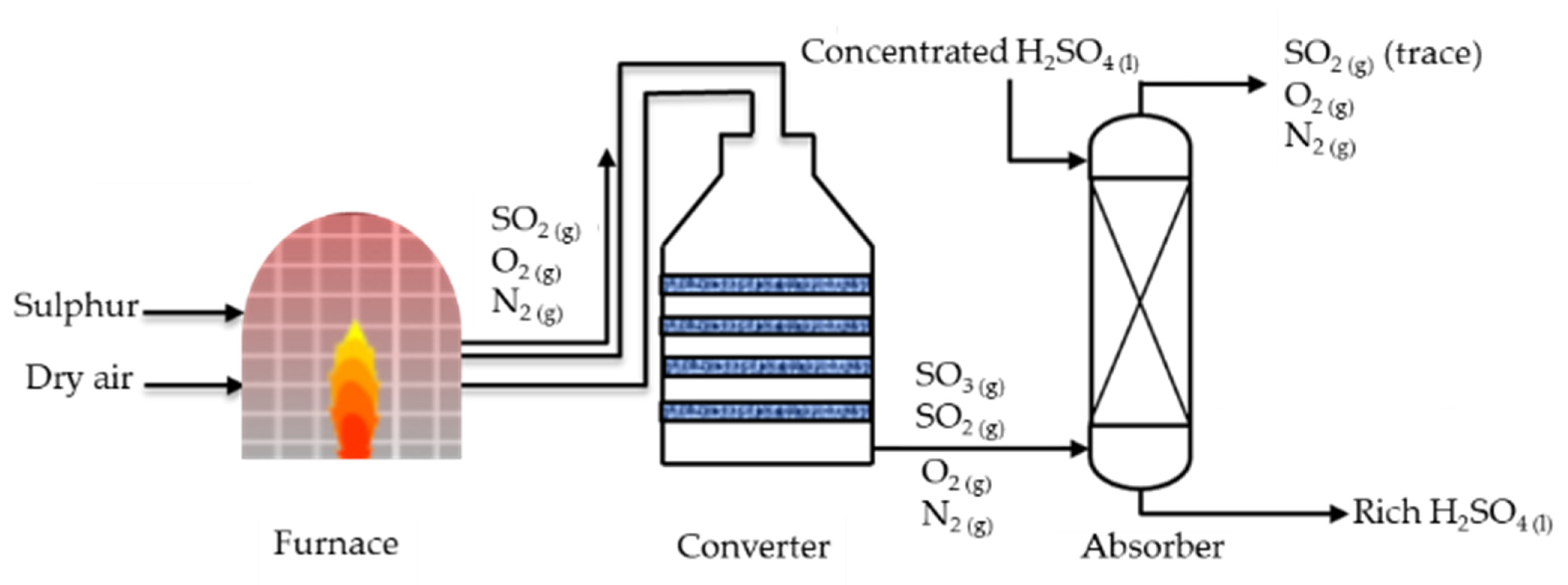
Processes | Free Full-Text | Modelling and Multi-Objective Optimization of the Sulphur Dioxide Oxidation Process

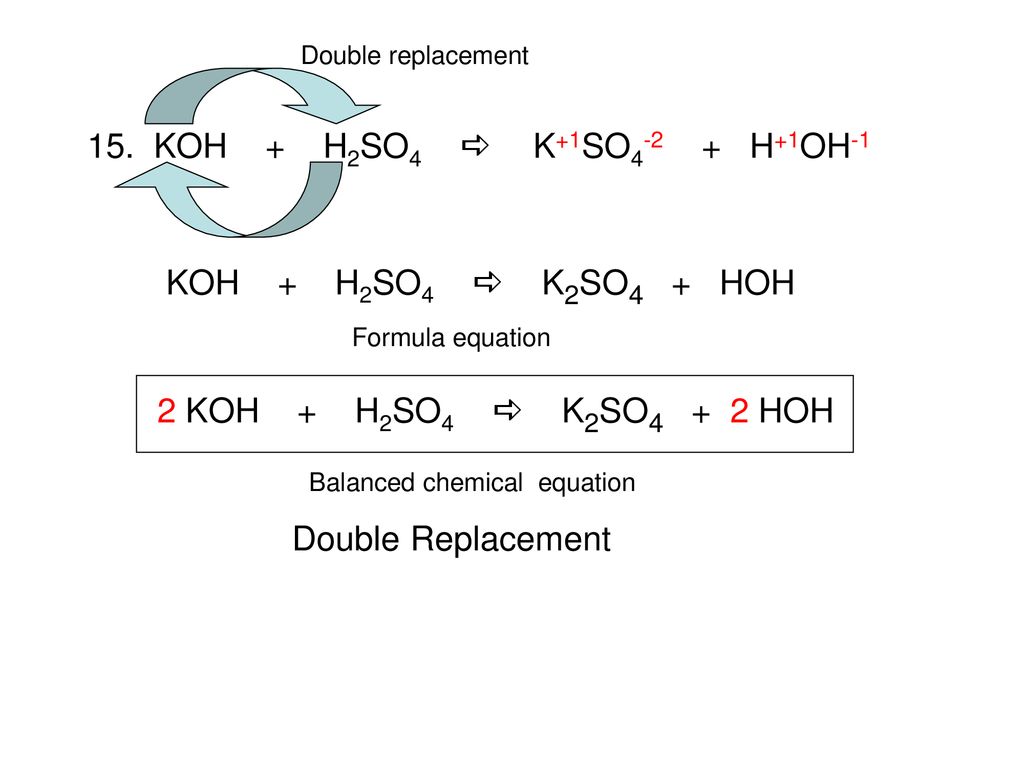
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) | Understanding, Balance, Molecules
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community

Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) | Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) Hello Everyone! Welcome back to